In this article, we are going to shed some light on the hotly discussed topic of how and why the air-fuel ratio and ignition timing affect horsepower.
And this is how we’re going to do it…
First, we are going to need some hard data. Using our test vehicle, we are going to do three power runs on our chassis dyno. In those three power runs, we are going to alter nothing except the target air to fuel ratio.
With that data up on the dyno screen, we are going to analyze the difference in performance from changing nothing more than the volume of fuel that is being delivered to the engine.
We will then do another set of three dyno runs, but this time keeping the air to fuel ratio consistent and vary the ignition timing by 2 degrees.
Air-Fuel Ratio and Horsepower
Here we have 3 dyno runs, back to back with no changes to anything except the air to fuel ratio.
Note how underwhelming the power gains are. Three completely different air-fuel ratios yielded almost identical power.
So why the big fuss with air-fuel ratio if they all make the same power and more practically – what air-fuel ratio should you be targeting for your engine?
To understand this we need to dig a bit deeper into the relationship between the air-fuel ratio and horsepower.
It’s all relative
What we see on the dyno graphs seems a bit counter-intuitive since the power output is only very loosely related to how much fuel we put into the engine. We can safely assume there is a fairly wide range of air to fuel ratios that we can run this engine at and make exactly the same power.
Sure if we go too far out on the rich side we will start to lose power. We’d probably also start to foul the spark plugs and put fuel into the oil as well.
We’ll get similar results when we start to lean out the engine. We’ll start losing power, though rather than fouling the spark plugs and diluting the engine oil, we will eventually melt pistons and cause catastrophic engine failures.
NOTE: On a turbocharged engine like the one in our test car, we could start leaning the engine out under full power to a point where we risk damaging it before we see any drop in horsepower. Take our word for it and don’t try it at home!
Meanwhile, inside your engine…
Just before we set off the spark plug, we have a mixture of air and fuel that are about to combine in a chemical reaction we call “combustion”.
Combustion produces heat. Heated air expands, but in our combustion chamber, there is no way for the air to expand to except down. Why down? There is only one movable thing inside the combustion chamber and that’s the piston. As the chemical reaction of combustion takes place, more and more heat is generated as we combine more and more of the air with the fuel inside the cylinder.

Eventually, we create enough pressure to push the piston down the bore. From here, the piston’s connected to the rod, the rod’s connected to the crank, the crank’s connected to… the burnouts!
The chemistry of combustion
Let’s take a closer look at the process of combustion. Like in any chemical reaction there is a specific ratio of the components that ensures we get a complete reaction. An excess of any one of the components just doesn’t get used.
In the case of our engine, the two components needed to complete our chemical reaction are air and fuel. One of those two components however is limited – the air. Why? There is only so much air we can stuff into our engine (if you haven’t read our article on Volumetric Efficiency do so now, because Volumetric Efficiency is all about how much air can be stuffed into an engine).
So we have a fixed amount of air available for our combustion reaction and we use the ECU to calculate the amount of fuel we need to put into the engine to match that incoming air.
Stoichiometric AFR
If we go back to the chemistry for gasoline the ideal amount of fuel we should be delivering to the engine for a complete chemical reaction is roughly 14.7 parts air to 1 part fuel by mass. We call this the Stoichiometric Air to Fuel Ratio. If we provide the engine with exactly 14.7 parts air to 1 part fuel all of both the air and the fuel get used up completely in the chemical reaction of combustion.
Of course, we know from experience that if we do this at 15 pounds of boost and 8000 RPM we stand a good chance of blowing the engine into oblivion and eating the pistons for lunch. However, if we just throw in a bit more fuel and run it at 12.5 parts air to 1 part fuel, the engine will happily run for hundreds of thousands of miles, making exactly the same horsepower.
Here is the curious thing though; in both cases, the engine is making the same horsepower, so it must be generating the same amount of heat, so why would one AFR result in melted internals and the other not?
Feeling the heat
It all comes down to that extra fuel. That additional fuel that we added actually cooled the combustion chamber from the inside preventing the excess heat build-up that causes the pistons to melt when the engine runs too lean.
When we say “too lean: we don’t mean “lean of the Stoichiometric 14.7 value”, we are talking about lean of the point where you cause engine damage, which on a turbo or supercharged engine is well before 14.7:1.
So we can conclude that horsepower is generated by producing heat but – the more heat you produce, the more fuel you need to add in order to cool the combustion chamber.
This is exactly why if you look at the target air-fuel ratio map in your software, the target gets richer with RPM and richer with manifold pressure.
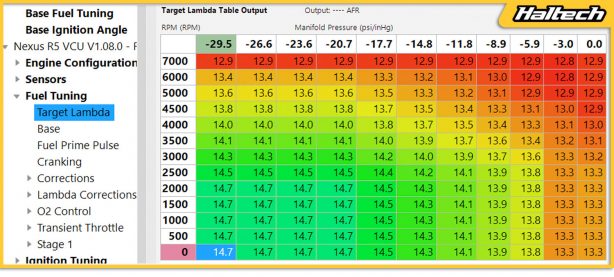
It gets richer with RPM because you are producing the heat more often. It gets richer with manifold pressure because manifold pressure represents how much air is going into the engine.
I can see clearly now
Let’s go back to the dyno graphs. All three runs have different air-fuel ratios but are all making the same power. This now makes sense, because we know we put the same amount of air in each of these runs so we could only ever hope to produce this amount of power from the combustion reaction.
The only difference in these runs would be in the engine’s internal combustion chamber temperatures. If we were able to measure these we would find that the temperature inside the chamber went down to a point where we reduced the combustion chamber temperature so much, we actually lost power. The trick here is knowing which of these air-fuel ratios delivers the best balance of engine longevity, fuel economy, and maintenance intervals.
NOTE: On a turbo engine like the one in our test car you should err on the side of caution and under full power the engine should be at the richest air-fuel ratio possible, whilst still maintaining peak performance. This would be transitioned to leaner mixtures as the engine load and RPM decreased.
Ignition Timing and Horsepower
For the second part of our demonstration we will run the car a couple more times, but this time we won’t touch the air-fuel ratio – instead, we will change the ignition timing.
The dyno graph clearly shows that just 2 degrees of timing shift cause significant changes to the output power. The difference between the two runs here was 20kW. while keeping the same air-fuel ratio and the same boost.
How does that happen? If there is no more or less heat being generated and we have the same amount of air and fuel in the reaction why are we making more power? To understand this we need to shift from chemistry to physics.
Let’s get physical
The engine’s rotating assembly is called just that because all its parts are moving. Rotating to be exact. This is important because if we apply the same amount of pressure to the crankshaft when it’s further around its rotation we get more leverage with the same force.

It’s important to note here that combustion doesn’t happen instantly. Once we light the fire at the spark plug, the fire spreads over a period of time. During that timeframe, the crankshaft is rotating and the piston is moving down. We need to allow for that delay when setting our ignition timing.

This is why as RPM increases we have to start the fire earlier and earlier (ie put more advance in the ignition timing map) giving the fire enough time to spread and create peak cylinder pressure at that same angle after TDC that creates maximum mechanical advantage on the crank.
There are so many competing factors going on with the ignition timing there really isn’t a reliable way to map your ignition timing correctly without using a dyno. You need to be able to actually receive real-time feedback on whether starting the spark earlier or later produced more or less mechanical advantage in a dynamic environment.
But the dyno graphs clearly show that with the same amount of air and the same amount of fuel (therefore generating the same amount of heat) we were able to get more power by simply moving the spark event to a time where it gained more mechanical advantage.
The verdict
The question on all the internet forums is “which is more important – ignition timing or AFR?” and just looking at the dyno graphs from our two experiments you could be forgiven for thinking the ignition timing has it. But if you’ve watched the video and read this article in full you’ll understand that it’s a bit more complicated than that.
The truth is – they are both equally important because they serve different purposes. The air-to-fuel ratio is used for thermal management, it’s there to ensure the engine produces just the right amount of heat to operate. The ignition timing is used to optimize the mechanical advantage within the engine’s cycle to produce power more efficiently.






Leave a comment